Recently, Gastroenterology published an interesting review, entitled “Pathobionts in Inflammatory Bowel Disease: Origins, Underlying Mechanisms, and Implications for Clinical Care”, by Ashley Gilliland and coworkers (GE 2024;166:44–58).
Herewith a brief summary. The gut microbiota plays a significant role in the pathogenesis of both forms of inflammatory bowel disease (IBD), namely, Crohn’s disease (CD) and ulcerative colitis (UC). Although evidence suggests dysbiosis and loss of beneficial microbial species can exacerbate IBD, many new studies have identified microbes with pathogenic qualities, termed “pathobionts,” within the intestines of patients with IBD. The concept of pathobionts initiating or driving the chronicity of IBD has largely focused on the putative aggravating role that adherent invasive Escherichia coli may play in CD. However, recent studies have identified additional bacterial and fungal pathobionts in patients with CD and UC. This review will highlight the characteristics of these pathobionts and their implications for IBD treatment. Beyond exploring the origins of pathobionts, we discuss those associated with specific clinical features and the potential mechanisms involved, such as creeping fat (Clostridium innocuum) and impaired wound healing (Debaryomyces hansenii) in patients with CD as well as the increased fecal proteolytic activity (Bacteroides vulgatus) seen as a biomarker for UC severity. Finally, we examine the potential impact of pathobionts on current IBD therapies, and several new approaches to target pathobionts currently in the early stages of development.
The following picture is taken from the article and depicts some of the proposed new therapies for IBD, specifically targeting the pathobionts.
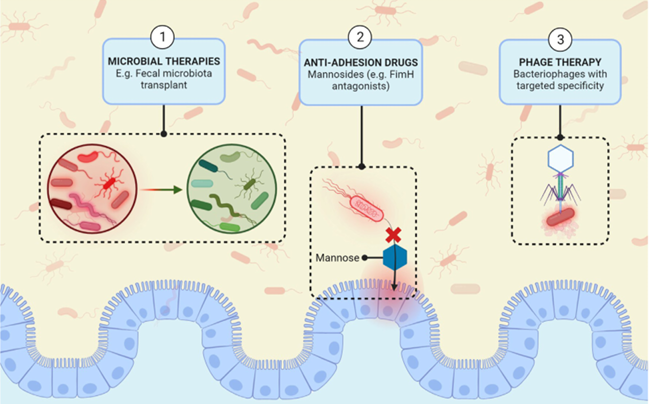
In summary, the article is interesting because it sheds some light on the specific kind of dysbiosis present in IBD, which is characterized not only by a reduced microbiota diversity and lower levels of anti-inflammatory bacteria, but also by an increase in potentially harmful microbes, called pathobionts.
Connected with the theme of pathobionts in IBD, I found particularly interesting a commentary on the March issue (Gastroenterology 2025;168:439–443) by N. Andreani & D. Unterweger [from the University of Kiel, Germany, and Max Planck Institute, respectively] introducing the concept of Evolutionary Medicine in the origin of the specific dysbiosis that leads to IBD.
Briefly, they analyzed four recent experimental studies showing evolutionary adaptation of gut bacteria to intestinal inflammation within weeks; such changes increase bacterial virulence in individuals with intestinal inflammation. The authors state that “an analysis of the intestinal microbiome of patients with IBD identified inflammation adapted bacterial lineages that evolved over millions of years”. They conclude that “these findings lay the foundation for evolutionary medicine in patients with chronically inflamed intestines. We see the rise of a new era in gastroenterology therapy with the potential for novel, evolution-informed treatment options ahead”.
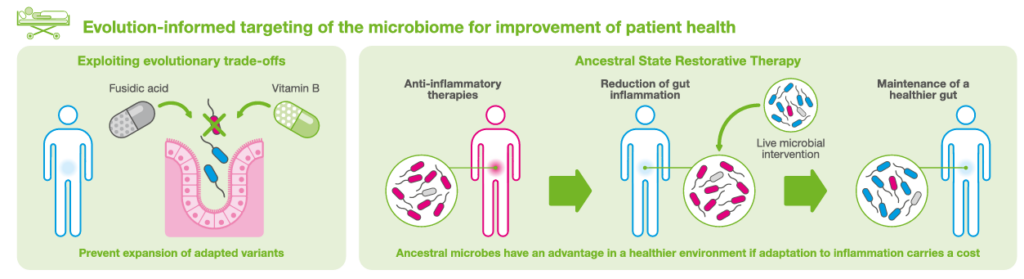
The interesting concept here, in my eyes, is the fact that specific pathobionts are able to evolve in the presence of intestinal inflammation, as summarized in the figure below
and that the process is potentially reversible at an early phase. Again, a therapy directed against these pathogens includes a microbial therapy (Probiotics), according to the scheme below.
The problem is how to identify in an early phase a cohort of potential patients.
A possible answer comes from another study published in Gastroenterology this month (GE 2025;168:99–110) by Pablo A. Olivera et al, showing that unaffected first-degree relatives (FDRs) from families with ≥ 2 affected FDRs with Crohn’s disease (CD, multiplex families) have a 3-fold risk of developing CD, and that this risk may be quantified by a score developed by authors. Interestingly, among the 4051 participants from simplex families and 334 from CD multiplex families. CD multiplex status was significantly associated with higher base line Fecal Calprotectin (P = .026) but not with increased gut permeability, as assessed by the lactulose-to-mannitol ratio. Three bacterial genera (one belongs to Prevotella genus) were found to be differentially abundant between both groups.
In my eyes, the studies I quoted are relevant to future investigation in the field of probiotics development and probiotics therapy. Those probiotics should be directed against specific pathobionts and possibly delivered to unaffected relatives of patients with IBD.
We can consider that this could only be a niche, although a very relevant one. But, despite the relatively low prevalence of IBD (in the USA, the incidence of inflammatory bowel disease (IBD) is estimated to be 721 cases per 100,000 people; this means that more than 0.7% of Americans have IBD, or almost 1 in 100), the number of relatives may be increased by a factor of 2, 3 or more, making the niche highly relevant.
🖋️ Fabio Pace
Director at Microbiota Center, ICH, Rozzano (Italy)