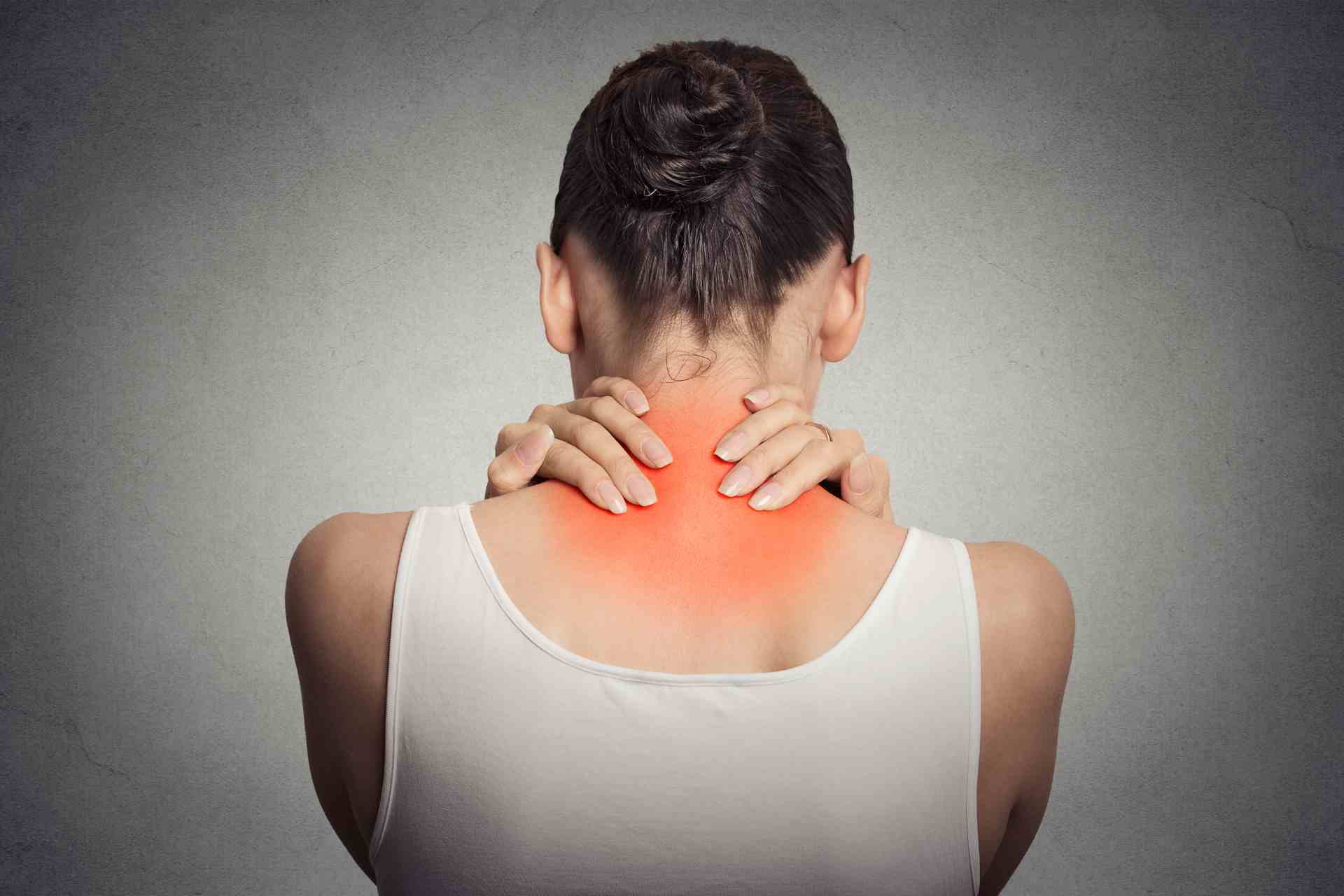
Fibromyalgia is a chronic pain condition that affects 2 to 4% of people, mostly women, causing pain, fatigue and cognitive issues. A new study shows that gut microbes play a key role in fibromyalgia by influencing pain, immune activity and metabolism.
The findings, published in Neuron, suggest that modifying the gut microbiota through fecal microbiota transplant from healthy individuals could offer a promising approach to reduce symptoms in people with fibromyalgia.
The cause of this chronic condition is unclear, but recent research shows that people with fibromyalgia have distinct gut bacteria, suggesting that the gut microbiota may play a role in their symptoms.
To better understand the impact of gut microbes on fibromyalgia, researchers led by Weihua Cai at McGill University took stool samples from women with the condition and from healthy women, and then transplanted them into germ-free female mice.
Pain response
The mice that received microbiota from women with fibromyalgia showed signs of increased sensitivity to pain—such as sensitivity to touch, heat, cold, and pressure—while mice given healthy microbiota did not. These changes in pain response lasted for at least four months and were accompanied by depression-like behaviors.
Mice that received microbiota from women with fibromyalgia showed altered levels of chemicals related to pain, as well as changes in how fats and bile acids were metabolized.
The mice also showed signs of inflammation and changes in immune cells, including an increase in certain inflammatory cells. In the spinal cord, specific immune cells called microglia became overactive—a phenomenon that is often seen in chronic pain. However, when the researchers replaced the microbiota of these mice with bacteria from healthy people, the animals’ symptoms improved.
Therapeutic approaches
Next, the team conducted a small study to test whether transplanting gut bacteria from healthy women into 14 women with severe fibromyalgia could reduce their symptoms.
Most participants showed improvements in pain, fatigue and overall quality of life. Their gut microbiotas began to resemble those of the healthy donors, and they showed changes in the levels of bacteria-derived chemicals that have been linked to pain and inflammation.
Although more rigorous testing is needed, the findings highlight the gut microbiota as a potential target for future fibromyalgia treatments, the authors say. The work, they add, “should facilitate the evaluation of such therapeutic approaches for this prevalent chronic pain syndrome.”