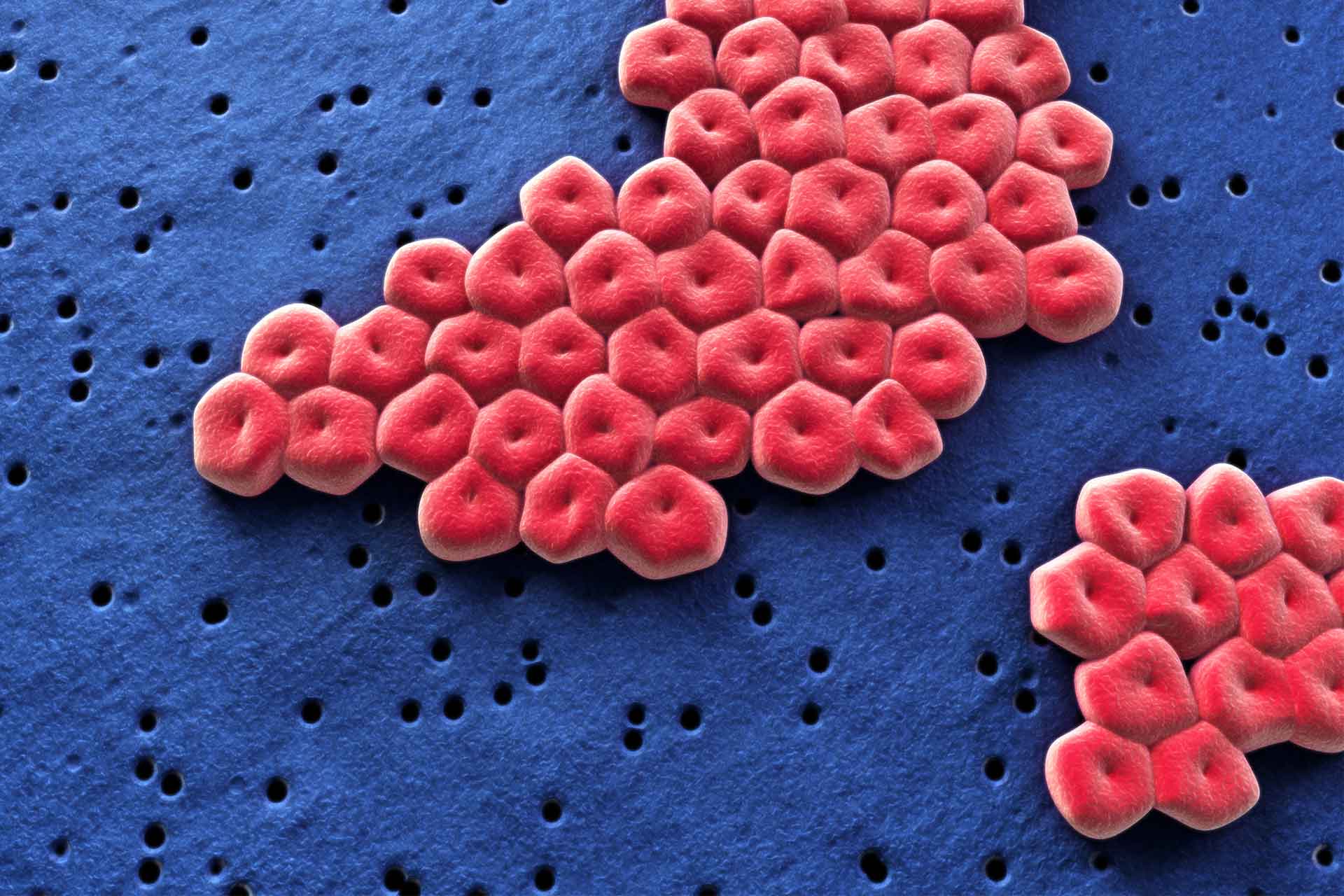
Acinetobacter baumannii is a dangerous antibiotic-resistant microbe that is rare in healthy people but common in hospitalized patients, especially after antibiotic treatment. Now, researchers have uncovered how A. baumannii is able to colonize the gut. The work also suggest that dietary protein and amino acids can promote its persistence.
The findings, published in Cell Host & Microbe, reveal a key strategy used by A. baumannii to colonize the gut and persist in it, highlighting a potential target for preventing its spread in healthcare settings.
Past studies showed that A. baumannii can persist in the gut, but little is known about its colonization strategies and its ability to compete for nutrients with other gut microbes. So, researchers led by Xiaomei Ren at the University of Illinois Chicago set out to investigate how A. baumannii colonizes the gut after antibiotic treatment in mice.
Gut colonization
The team found that A. baumannii uses a special metabolic pathway to break down the amino acid ornithine. This pathway is found in most harmful strains of the bacteria but not in harmless ones.
The team also identified a key gene, called AstO, that enables A. baumannii to use ornithine as a food source, helping it to survive and compete with other bacteria in the gut antibiotics disrupt the normal gut microbiota. Bacteria with the AstO gene were able to stay in the mice’s gut much longer than those without it, the researchers found.
When the animals were given extra ornithine in their drinking water, the bacteria colonized their guts for longer, suggesting that ornithine from the diet can help A. baumannii persist. Further analyses showed that antibiotic treatment changes the gut’s amino acid environment, making it easier for A. baumannii to establish itself, but the bacteria must use ornithine to maintain their presence.
Dietary influence
Next, the researchers analyzed stool samples from infants aged 1 to 24 months and found that A. baumannii is most abundant between 1 and 4 months, then decreases as the gut microbiota becomes more diverse. Formula-fed infants had higher levels of A. baumannii at 4 months compared to breastfed infants, the team also found.
This result suggests that diet might influence the ability of A. baumannii to colonize the infant gut. Understanding how this microbe adapts to compete with the gut microbiota could lead to new ways to prevent it from establishing itself and causing infections in hospitals, the authors say.
The findings, they add, “have significant clinical relevance, as gut colonization is thought to be important [for anti-microbial resistance] reservoir in healthcare facilities.”