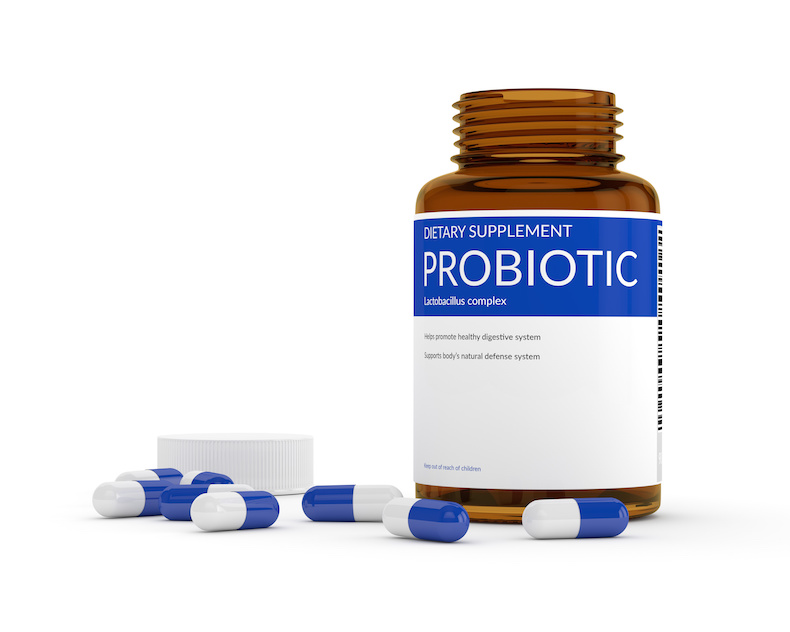
AESAN, the Spanish Agency for Food Safety and Nutrition, says the term ‘probiotic’ will be allowed on labels of food products, infant formula, and supplements on sale in Spain, providing it is not accompanied by any health claims.
A report published by AESAN notes that the lack of harmonised regulations around the labelling of probiotics in Europe mean that according to the principle of mutual recognition – which guarantees that any good lawfully sold in one EU country can be sold in another – the term ‘probiotic’ may be used on food labels in Spain, so long as the wording is not accompanied by specific health claims that are not authorised under European health claims regulations.
While there is no European legal framework defining the probiotic category, or providing a harmonised framework for the sale of probiotics in Europe, a decision by the European Food Safety Authority (EFSA) and the European Commission (EC) that the term probiotic is itself an implied health claim means that it falls foul of EC regulation 1924/2006 on health claims and cannot be used on-pack.
According to the Commission: “the term implies that a product provides a health benefit, which could be misleading to consumers unless it can be scientifically substantiated.”
Yet multiple interpretations of the EC guidance by European nations, and specific allowances for the use of the term in countries like Italy mean that the Spanish regulator now says the term should be allowed under the principle of mutual recognition.
“In the field of food supplements, it has been found that there are a large number of food supplements on the market, which include the term ‘probiotic/s’. These products come from different EU countries, where they are allowed to be marketed under this name and, therefore, they could not be prevented from being marketed in Spain, in application of the ‘principle of mutual recognition’ established in the Treaty of the European Union,” wrote AESAN in its report.
Commenting on the report, Luis Gosálbez Managing Director at Sandwalk BioVentures, said the capacity to use the term ‘probiotic’ on product labels is “a small victory for the microbiome industry.”
Lack of harmonization
The Spanish regulator noted that there is no ‘specific legislation’ that regulates the use of probiotics in human food. As such, it says there are no specific requirements for them, nor is there a list of authorized probiotics – adding that in the absence of a list of authorized microorganisms at the European Union level, the QPS list of the European Food Safety Authority (EFSA) is taken as a reference for their safe use in food.
AESAN adds that food supplements containing one or more strains of live microorganisms are marketed in the European Union, as are infant formulas and follow-on formulas. Furthermore, in some foods such as yoghurt, probiotic strains are essential for manufacture. In all cases, it notes that products comply with the safety requirements.
“From the discussions that have been held within the European Commission’s group of experts on nutritional and health claims, it is found that there are different interpretations by Member States regarding the use of the term ‘probiotic’, which, in turn, implies a non-harmonized situation in the European Union market,” says the report – adding that since many food products and nutritional supplements are from other European countries that do allow the term ‘probiotic’ to be used, then mutual recognition laws mean that use of the term cannot be prevented.
AESAN added that until there is a ‘uniform criterion’ relating to the use of the term ‘probiotic’ in Europe it will be considered to be acceptable that the word appears on the label of foods and nutritional supplements. However, it reiterated that the use of the term probiotic should not be accompanied by any health claims, unless expressly authorized under European health claims regulation.