• Nitrogenous Wastes
• Bacterial Microbial Ecosystem
What is already known on this topic
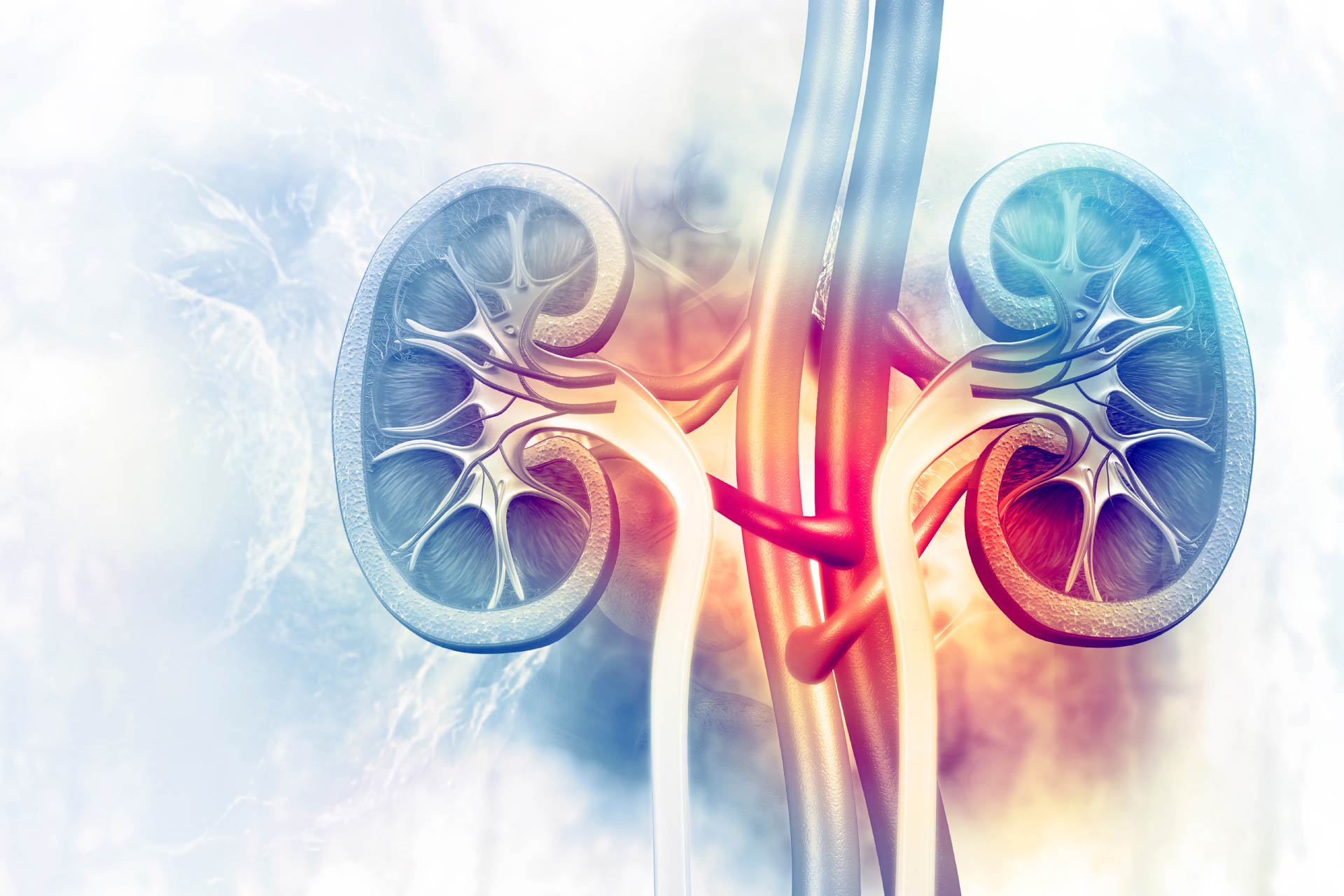
Healthy kidneys filter the blood and rid the body of nitrogen-containing waste products such as urea, creatinine, and ammonia from normal metabolic activities. Patients with acute kidney injury or end stage kidney disease typically require dialysis, which is invasive and carries a risk of infection and other complications.What this research adds
Researchers isolated three strains of mouse gut bacteria that work together to degrade urea and creatinine and recycle them into amino acids without a toxic buildup of ammonia. They housed the microbes in engineered microcapsules that kept the bacteria close to one another and limited access to the target nitrogenous waste products.Conclusion
When administered orally in the engineered microcapsules, the three gut bacteria strains improved survival, recovered kidney function, and reduced buildup of nitrogen-containing waste products in animal models of acute kidney injury and chronic kidney failure. As this biotechnology develops further and is adapted to humans, it could improve quality of life and outcomes for dialysis patients.
Healthy kidneys filter the blood and rid the body of nitrogenous waste products such as urea, creatinine, and ammonia from normal metabolic activities. Patients with acute kidney injury or end stage kidney disease typically require dialysis to perform this function. Now researchers have identified three strains of mouse gut bacteria that work together to degrade urea and creatinine and recycle them into amino acids without a toxic buildup of ammonia. They housed the microbes in engineered microcapsules that kept them close to one another and limited access to the target nitrogenous waste products.
The results, published in Nature Biomedical Engineering, demonstrate that when administered orally in engineered microcapsules, the isolated microbial strains improved survival, recovered kidney function, and reduced nitrogenous waste products in animal models of acute kidney injury or chronic kidney failure. As this biotechnology develops further and is adapted to human patients, it could improve quality of life and outcomes for dialysis patients.
Previous research into the treatment of kidney injury and disease explored biotechnology solutions and antioxidant drugs, but the biotechnology approaches are invasive, and the drugs have yielded ambiguous results. Development of a safe, effective, non-invasive treatment to reduce the need for dialysis would alleviate significant health burdens for patients. The influence of the gut microbiota on host health is well documented, and the ease of manipulating gut microbial populations with oral supplementation presents therapeutic options.
Nitrogenous Wastes
Di-Wei Zheng, Pei Pan, et. al. analyzed the in vitro metabolic activity of microbiota from fecal samples derived from four types of mice. They isolated two strains that demonstrated a high capacity to convert either urea or creatinine, nitrogenous waste products, into ammonia. Since ammonia is highly toxic, a third bacterial strain which effectively converted ammonia into amino acids was combined with the other two to form an artificial microbial community.
Bacterial Microbial Ecosystem
To explore the in vivo metabolic activity of the three microbial strains, the researchers engineered a bacterial micro-ecosystem (“BME”) to encapsulate the microbes in microspheres with a semi-permeable nanofilm on the surface. The microspheres assured proximity of the microbial strains to one another within the artificial ecosystem enabling the bacteria to function cohesively rather than allowing them to freely colonize different regions of the host gastrointestinal tract. The nanofilm coating permitted entry to the BME by smaller nitrogenous waste molecules targeted for degradation while preventing access to – and thereby protecting from breakdown – larger proteins.
After first testing the safety profile, the researchers administered the BME to mice and miniature pigs previously subjected to induced acute kidney injury or chronic kidney failure. The animals treated with the BME exhibited higher survival rates and superior blood urea and creatinine clearance compared to animals treated with either peritoneal dialysis or free unencapsulated bacterial strains. Diagnostic imaging further showed a quick recovery of kidney function in the BME treatment group while the control and dialysis groups exhibited impaired kidney function following the chemical insults. The dialysis group recovered some kidney function over time but exhibited inflammatory changes to the peritoneal membrane.
This research demonstrated that oral administration of three gut bacterial strains contained in engineered microcapsules reduced the workload on damaged kidneys and prolonged survival in the study animals with no adverse effects. A human analog could potentially reduce the need for dialysis.