• The gut microbiota’s role in immune function
• The effects of antimicrobials on microbiota and immune system
-
What is already known on this topic
The composition and function of the gut microbiota is influenced by a series of factors, including our exposure to antimicrobials. Besides altering the gut microbiota, antimicrobials can reduce the protective effects of commensal microbes against pathogens and foster the development of antimicrobial resistance. -
What this research adds
This Perspective article summarizes how the gut microbiota influences host immunity, and it describes strategies to manage antimicrobial resistance. -
Conclusions
Manipulating the gut microbiota to reduce the number of antibiotic-resistant microbes or developing drugs that have protective effects against pathogens may help to reduce the use of antibiotics and the spread of antimicrobial resistance, the authors say.
Antimicrobial resistance – the ability of a microbe to resist the effects of a drug that once could successfully treat the microbe – is one of the biggest public health challenges of our time. To manage it and enhance protective immune responses against pathogens, a duo of scientists proposes to turn to the gut microbiota.
David Relman of Stanford University School of Medicine, California, and Marc Lipsitch of Harvard T. H. Chan School of Public Health in Boston, Massachusetts, published a Perspective article in Proceedings of the National Academies of Sciences, where they summarize how the gut microbiota influences host immunity and describe strategies to control antimicrobial resistance.
The gut microbiota’s role in immune function
Gut microbes, in particular specific bacterial families such as Clostridium species, are key for immune function. Several studies have shown that the development and differentiation of the immune system of animals born in the absence of microbes is incomplete. Immune system development and differentiation is completed only when the animals are colonized with a gut microbiota from their own species.
The influence of gut microbes on the immune system likely begins before birth. Experiments in mice suggest that the presence of specific immune cells in pups, which prevent gut inflammation, is influenced by which microbes colonize the gut of the pups’ mother.
After birth, the transfer to the newborn of antibodies such as IgA and IgG through breast milk provides protection against pathogens. Later in life, when the newborn develops its own immune responses, bacterial metabolites such as short-chain fatty acids can influence IgA production and promote the differentiation of immune cells in the gut.
In addition to metabolites, components of the microbes’ cell-wall and other microbial molecules have been shown to regulate the function of the immune system and the gut barrier.
The effects of antimicrobials on microbiota and immune system
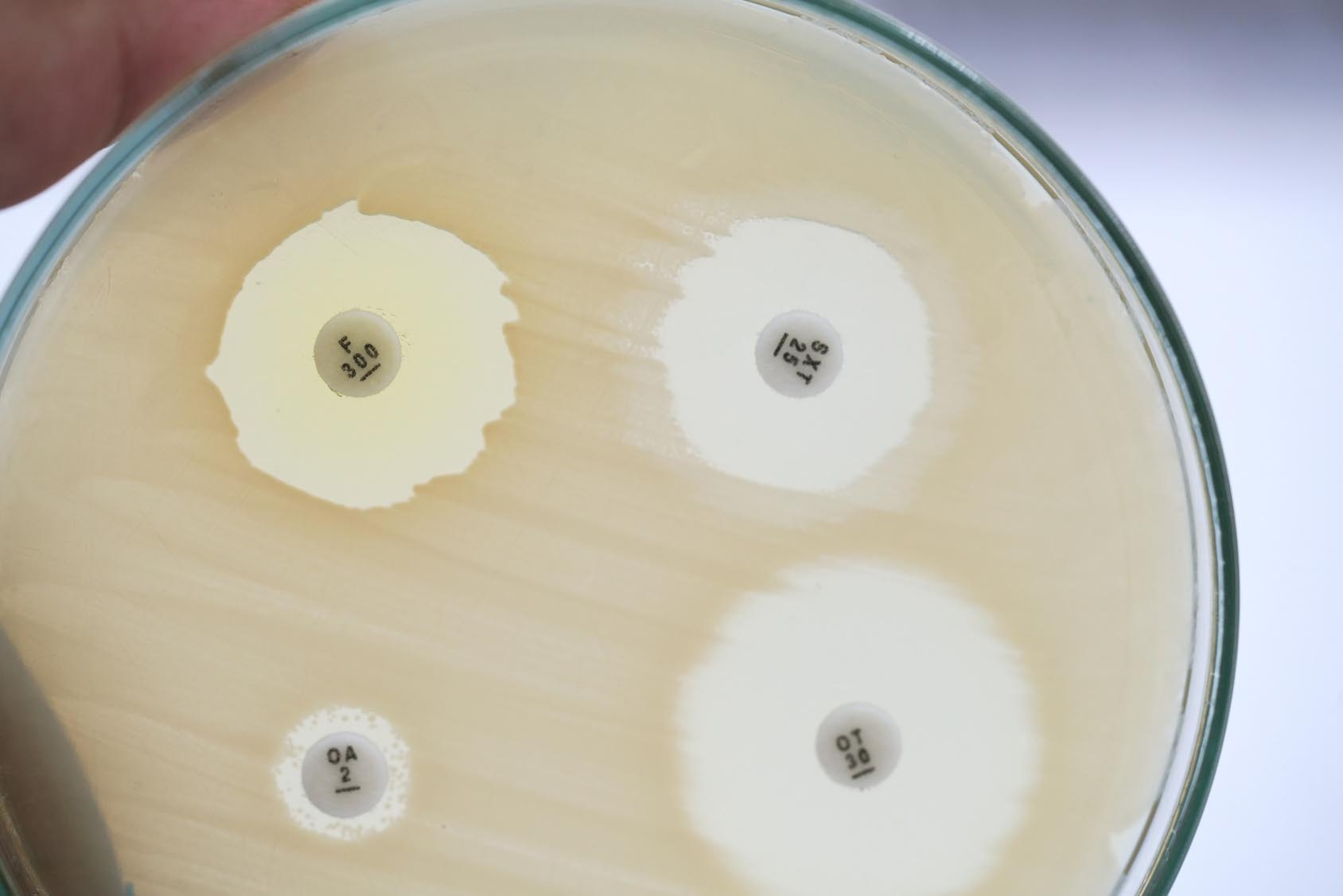
The use of antibiotics in children and adults could reduce the protective effects of commensal microbes against pathogens and alter the gut microbiota composition. For example, the structure of the fecal microbiota of people taking the antibiotic ciprofloxacin for 5 days changed rapidly, with 25% to 50% of the bacterial species, including Ruminococcaceae and Bacteroides, dramatically decreasing in abundance.
In babies born to women who receive antibiotics during delivery, not only the gut microbiota composition but also the production of bacterial metabolites such as short-chain fatty acids are altered during the first 3 months of life.
What’s more, antibiotics can foster the development and spread of antimicrobial resistance. A study that analyzed fecal samples from more than 200 individuals living in the United States, Denmark, and Spain has found that resistance genes for antibiotics used in livestock were more abundant than those for antibiotics not used in animals.
A handful of studies suggest that antimicrobials could also influence vaccine efficacy. For instance, experiments in mice showed that immune responses to the influenza vaccine depend on the presence of commensal gut bacteria and on the bacterial protein flagellin, suggesting that the gut microbiota serves as an adjuvant for immunization.
Because of the interplay between the microbiota and the host’s immune system, the authors highlight two emerging strategies that could be used to manage antimicrobial resistance. The first approach is to directly manipulate the gut microbiota by delivering single strains or bacterial communities that improve or restore the protective effects against pathogens.
The second approach is to target specific pathogens and antibiotic-resistant microbes by developing vaccines or narrow-spectrum antimicrobial drugs.
Both strategies, the authors say, could reduce the use of broad-spectrum antibiotics and the number of antibiotic-resistant bacteria.